Modify methionine to show its zwitterion form – In the realm of biochemistry, the modification of methionine to reveal its zwitterionic form has garnered significant attention. Methionine, an essential amino acid, possesses a unique chemical structure that enables it to exist in a zwitterionic state, a form that carries both positive and negative charges.
Understanding this zwitterionic form is crucial for deciphering methionine’s interactions and functions within biological systems.
Delving into the intricacies of methionine modification, researchers have developed a repertoire of chemical techniques to alter its structure and properties. These modifications not only provide insights into methionine’s behavior but also pave the way for its application in various fields.
Introduction
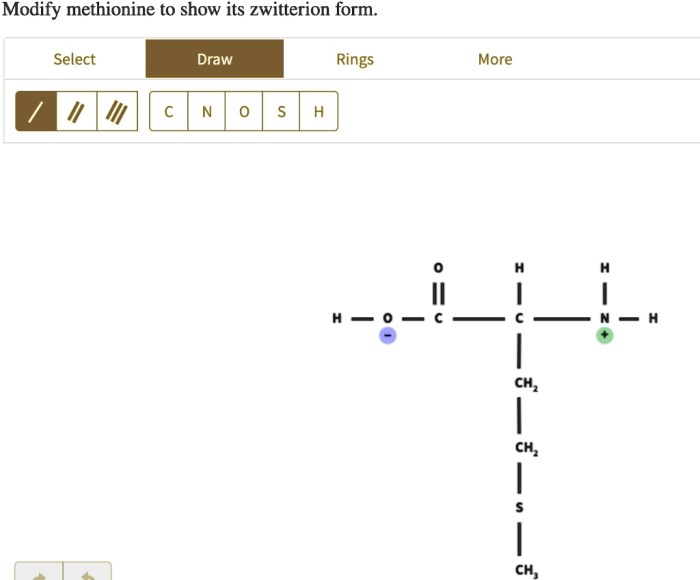
Methionine is an essential amino acid that plays a crucial role in various biochemical processes. Its unique chemical structure, characterized by a sulfur-containing side chain, endows it with distinctive properties. Understanding the zwitterion form of methionine is essential for comprehending its interactions and functions in biological systems.
Chemical Modifications of Methionine
Methionine can undergo a range of chemical modifications, including methylation, oxidation, and phosphorylation. These modifications alter the structure and properties of methionine, affecting its biological activity and interactions with other molecules.
Methylation
Methylation involves the addition of a methyl group to the sulfur atom of methionine. This modification is catalyzed by specific enzymes called methionine methyltransferases. Methylated methionine plays a role in protein synthesis, DNA methylation, and cellular signaling.
Oxidation
Methionine can be oxidized to form methionine sulfoxide, which can further undergo additional oxidation steps. Oxidation of methionine affects its stability and function, and has been implicated in aging and oxidative stress.
Phosphorylation
Phosphorylation involves the addition of a phosphate group to the hydroxyl group of methionine. This modification is catalyzed by kinases and plays a role in regulating protein function and signaling pathways.
Zwitterion Formation of Methionine
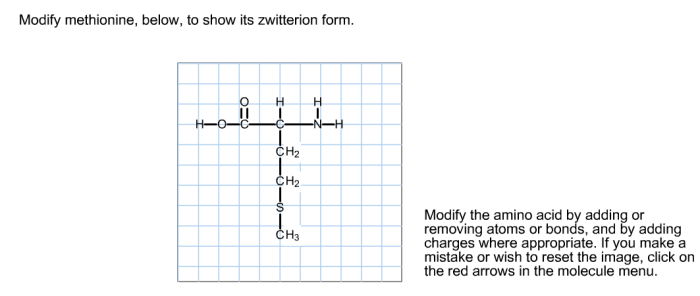
Methionine exists in a zwitterion form at physiological pH. This means that the amino and carboxyl groups of methionine are both ionized, resulting in a net neutral charge. The formation of the zwitterion is influenced by the pH of the environment.
Role of pH
At low pH (acidic conditions), the amino group of methionine is protonated (NH3+) and the carboxyl group is deprotonated (COO-), resulting in a cationic form of methionine. As the pH increases (more alkaline conditions), the amino group becomes deprotonated (NH2) and the carboxyl group becomes protonated (COOH), resulting in an anionic form of methionine.
Implications for Interactions, Modify methionine to show its zwitterion form
The zwitterion form of methionine has implications for its interactions with other molecules. The charged nature of the zwitterion allows it to form electrostatic interactions with other charged molecules, influencing its solubility, stability, and binding properties.
Applications of Methionine Zwitterion
The zwitterion form of methionine has various applications, including:
- Protein crystallization:Methionine zwitterion can promote protein crystallization by facilitating protein-protein interactions and reducing aggregation.
- Drug delivery:Methionine zwitterion can be used as a carrier for drug delivery, improving drug solubility and stability.
- Cosmetics:Methionine zwitterion has antioxidant and moisturizing properties, making it a valuable ingredient in cosmetic formulations.
User Queries: Modify Methionine To Show Its Zwitterion Form
What is the significance of methionine’s zwitterionic form?
The zwitterionic form of methionine plays a crucial role in its interactions with other molecules. Its amphiphilic nature allows it to interact with both hydrophilic and hydrophobic environments, facilitating its incorporation into proteins and membranes.
How is the zwitterionic form of methionine formed?
The zwitterionic form of methionine is formed when the amino group accepts a proton (H+) and the carboxylic acid group loses a proton (H+), resulting in a net charge of zero.
What are the applications of methionine zwitterion?
Methionine zwitterion has applications in drug development, material science, and cosmetics. In drug development, it is used to improve the solubility and bioavailability of drugs. In material science, it is used as a building block for biocompatible materials. In cosmetics, it is used as a moisturizing agent.