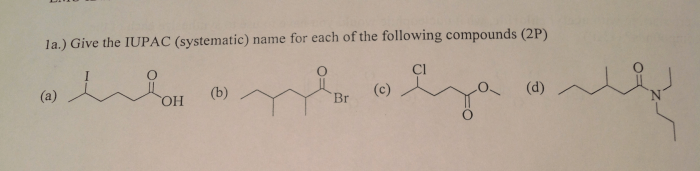
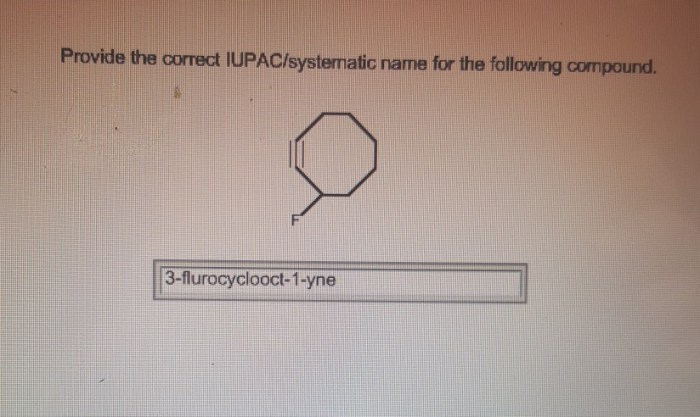
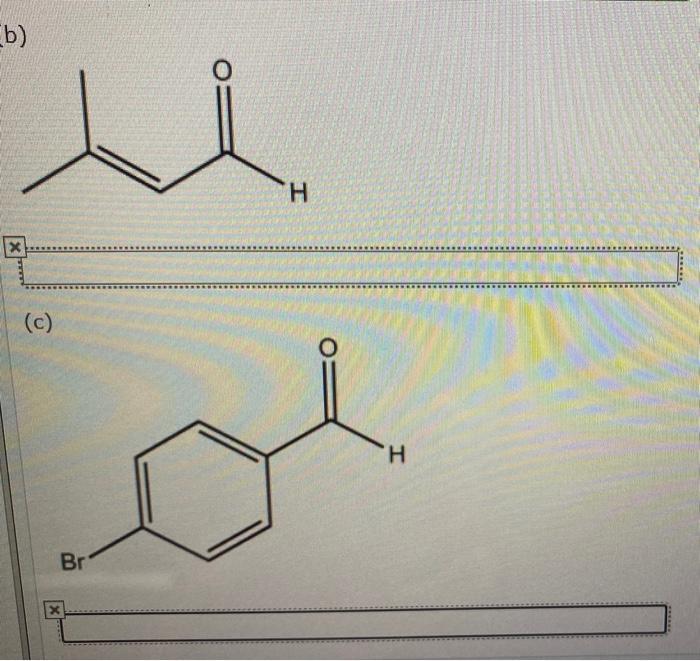
Give the systematic iupac name for the following. – Give the systematic IUPAC name for the following: this comprehensive guide delves into the intricacies of organic compound nomenclature, providing a structured approach to naming organic compounds according to the International Union of Pure and Applied Chemistry (IUPAC) guidelines. Embark on a journey of chemical precision as we explore the rules, conventions, and systematic naming of organic compounds, empowering you with the knowledge to accurately identify and name these essential molecules.
This guide will equip you with a deep understanding of functional groups, parent chains, substituents, numbering, prefixes, and suffixes, providing a solid foundation for mastering IUPAC nomenclature. Through a combination of clear explanations, illustrative examples, and practice exercises, you will gain the confidence to navigate the complexities of organic compound naming.
1. IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) nomenclature system is a standardized set of rules for naming organic compounds. It ensures that compounds have consistent and unambiguous names, regardless of their structure or complexity.
The IUPAC nomenclature system is based on the following principles:
- The parent chain is the longest continuous chain of carbon atoms in the compound.
- Substituents are groups of atoms that are attached to the parent chain.
- The name of the compound is based on the parent chain and the substituents.
2. Systematic Naming
To systematically name an organic compound using IUPAC nomenclature, the following steps are followed:
- Identify the parent chain.
- Identify the substituents.
- Number the parent chain.
- Assign prefixes to the substituents.
- Combine the prefixes and the parent chain name to form the name of the compound.
3. Functional Groups
Functional groups are groups of atoms that have characteristic chemical properties. They influence the IUPAC name of a compound because they determine the suffix that is used.
Some common functional groups include:
- Alkanes (-ane)
- Alkenes (-ene)
- Alkynes (-yne)
- Alcohols (-ol)
- Aldehydes (-al)
- Ketones (-one)
- Carboxylic acids (-oic acid)
4. Parent Chain
The parent chain is the longest continuous chain of carbon atoms in the compound. It is used to determine the base name of the compound.
When selecting the parent chain, the following criteria are used:
- The parent chain must contain the greatest number of carbon atoms.
- The parent chain must contain the most functional groups.
- The parent chain must have the lowest number of double bonds.
5. Substituents
Substituents are groups of atoms that are attached to the parent chain. They are named according to their structure and the number of carbon atoms they contain.
The following are some common substituents:
- Methyl (-CH3)
- Ethyl (-CH2CH3)
- Propyl (-CH2CH2CH3)
- Butyl (-CH2CH2CH2CH3)
- Halo (-F, -Cl, -Br, -I)
- Hydroxy (-OH)
- Amino (-NH2)
6. Numbering
The parent chain and substituents are numbered to indicate their position on the chain.
The following rules are used for numbering:
- The parent chain is numbered from one end to the other.
- The substituents are numbered according to their position on the parent chain.
- The lowest number possible is assigned to each substituent.
7. Prefixes and Suffixes
Prefixes and suffixes are used to indicate the number and type of substituents.
The following are some common prefixes and suffixes:
- Prefixes: mono-, di-, tri-, tetra-, etc.
- Suffixes: -ane, -ene, -yne, -ol, -al, -one, -oic acid
8. Examples and Practice, Give the systematic iupac name for the following.
Here are some examples of systematic IUPAC names for organic compounds:
- Methane
- Ethane
- Propane
- Butane
- Pentane
- Hexane
- Heptane
- Octane
- Nonane
- Decane
Here are some exercises to practice systematic IUPAC naming:
- Name the following compound: CH3CH2CH2CH2CH3
- Name the following compound: CH3CH2CH(CH3)CH2CH3
- Name the following compound: CH3CH2CH2CH2CH2OH
- Name the following compound: CH3CH2CH2CH2CHO
- Name the following compound: CH3CH2CH2CH2COOH
Questions and Answers: Give The Systematic Iupac Name For The Following.
What is IUPAC nomenclature?
IUPAC nomenclature is a systematic method of naming organic compounds developed by the International Union of Pure and Applied Chemistry (IUPAC). It provides a standardized and unambiguous way to identify and name organic compounds based on their structure and functional groups.
Why is IUPAC nomenclature important?
IUPAC nomenclature is important because it allows scientists and researchers to communicate about organic compounds in a clear and concise manner. It ensures that everyone is using the same naming conventions, reducing confusion and errors in communication.
How do I determine the IUPAC name of an organic compound?
To determine the IUPAC name of an organic compound, you need to follow a set of rules and conventions established by IUPAC. These rules consider the structure of the compound, the presence of functional groups, and the numbering of the carbon atoms.