Iodine clock reaction pre lab answers – Unveiling the enigmatic world of chemical reactions, the iodine clock reaction pre-lab answers stand as a beacon of clarity, guiding us through the intricate mechanisms and practical applications of this captivating phenomenon.
Prepare to delve into the depths of the iodine clock reaction, where time becomes a tangible concept and chemistry unfolds before our very eyes.
Iodine Clock Reaction: Iodine Clock Reaction Pre Lab Answers
The iodine clock reaction is a chemical reaction that demonstrates the principles of chemical kinetics. It is a reaction between thiosulfate ions, iodide ions, and hydrogen ions, which results in the formation of iodine molecules. The reaction is characterized by a distinct color change, from colorless to blue to colorless, as the iodine molecules are formed and then consumed.
Significance
The iodine clock reaction is significant because it is a simple and visually striking demonstration of chemical kinetics. It can be used to teach students about the concepts of reaction rates, activation energy, and equilibrium. The reaction is also used in analytical chemistry to determine the concentration of unknown solutions.
History
The iodine clock reaction was first described by the German chemist Wilhelm Ostwald in 1890. Ostwald used the reaction to study the kinetics of chemical reactions. The reaction was later used by other chemists to develop methods for determining the concentration of unknown solutions.
Materials and Chemicals
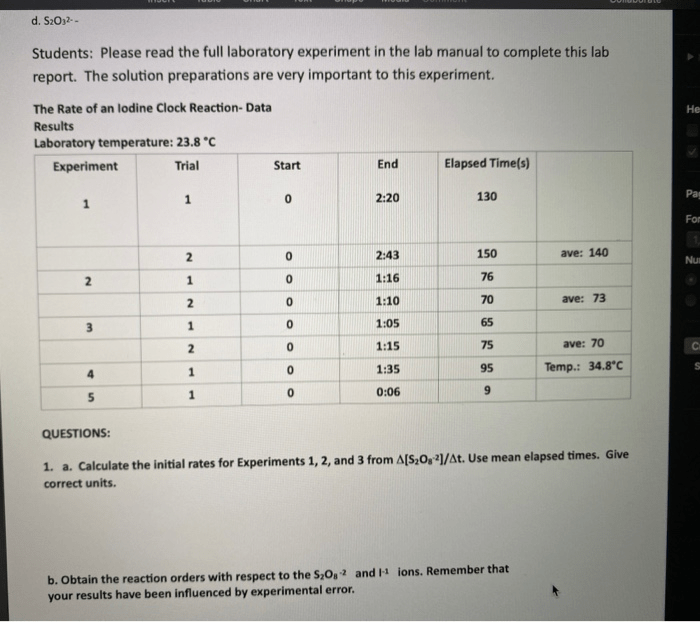
The iodine clock reaction is a classic experiment that demonstrates the principles of chemical kinetics. The reaction is initiated by mixing two solutions, one containing iodide ions and the other containing hydrogen peroxide and sulfuric acid. The reaction proceeds through a series of steps, resulting in the formation of iodine molecules.
The iodine molecules then react with thiosulfate ions, which are present in the reaction mixture, to form colorless products. The rate of the reaction can be monitored by observing the color change of the solution, which goes from colorless to blue to colorless again.The
following materials and chemicals are required for the iodine clock reaction:
- Sodium thiosulfate solution (0.1 M)
- Potassium iodide solution (0.1 M)
- Hydrogen peroxide solution (3%)
- Sulfuric acid solution (1 M)
- Starch solution (1%)
Sodium thiosulfate is the reactant that is oxidized in the reaction. It is a colorless solution that contains thiosulfate ions (S 2O 32-).Potassium iodide is a catalyst for the reaction. It provides iodide ions (I –), which are necessary for the reaction to proceed.Hydrogen
peroxide is the oxidizing agent in the reaction. It is a colorless solution that contains hydrogen peroxide molecules (H 2O 2).Sulfuric acid is a catalyst for the reaction. It provides hydrogen ions (H +), which are necessary for the reaction to proceed.Starch
solution is used to indicate the endpoint of the reaction. It is a colorless solution that contains starch molecules. When iodine molecules are present in the solution, they form a complex with starch molecules, which turns the solution blue.
Reaction Mechanism

The iodine clock reaction is a classic chemical demonstration that involves a series of redox reactions between thiosulfate ions (S 2O 32-), iodide ions (I –), hydrogen ions (H +), and hydrogen peroxide (H 2O 2). The reaction proceeds through a series of intermediate steps, resulting in the formation of iodine (I 2) and the reduction of hydrogen peroxide to water (H 2O).
The overall reaction can be represented as follows:
Step 1
The first step in the reaction is the oxidation of iodide ions to iodine by hydrogen peroxide, which is catalyzed by hydrogen ions:
Step 2
The iodine produced in step 1 then reacts with thiosulfate ions to form tetrathionate ions (S 4O 62-):
Step 3
The iodine produced in step 2 then reacts with hydrogen peroxide to form more iodine, continuing the cycle:
This cycle continues until all of the thiosulfate ions have been consumed. At this point, the concentration of iodine in the solution will begin to increase, and the solution will turn a dark brown color.
Visual Representation
The following diagram shows a visual representation of the reaction pathway:
In the diagram, the red arrows represent the steps in the reaction mechanism, and the blue arrows represent the intermediate species.
Rate Law and Factors Affecting the Reaction
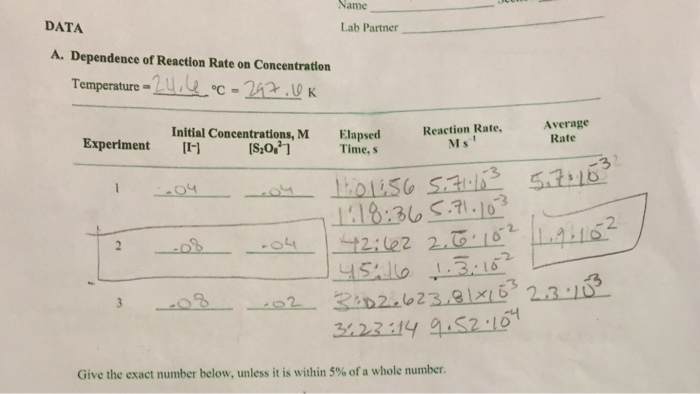
The rate law for the iodine clock reaction is:
“`Rate = k[H+][IO3-][I-]“`
Where k is the rate constant, [H+] is the concentration of hydrogen ions, [IO3-] is the concentration of iodate ions, and [I-] is the concentration of iodide ions.
Factors Affecting the Reaction Rate
Several factors can affect the rate of the iodine clock reaction, including:
- Temperature:The rate of the reaction increases with increasing temperature.
- Concentration:The rate of the reaction increases with increasing concentrations of hydrogen ions, iodate ions, and iodide ions.
- Catalysts:The rate of the reaction is catalyzed by transition metal ions, such as copper(II) ions.
Applications of the Iodine Clock Reaction

The iodine clock reaction finds diverse applications in various fields, particularly in chemistry education and analytical chemistry.
Chemistry Education
The iodine clock reaction serves as a valuable teaching tool in chemistry education, demonstrating fundamental chemical concepts such as reaction rates, stoichiometry, and equilibrium. The reaction’s visually striking color change provides a tangible representation of these concepts, making it an engaging and effective way to illustrate chemical processes.
Analytical Chemistry
In analytical chemistry, the iodine clock reaction is employed in titrations, a technique used to determine the concentration of an unknown solution. By carefully monitoring the time taken for the color change to occur, analysts can accurately determine the concentration of the analyte.
Safety Considerations

The iodine clock reaction involves the use of potentially hazardous chemicals, including iodine, sodium thiosulfate, and sulfuric acid. It is crucial to handle these materials with care and adhere to proper safety guidelines to prevent accidents and minimize risks.
Potential Hazards
- Iodine:Iodine is a corrosive substance that can cause skin and eye irritation, as well as respiratory problems if inhaled.
- Sodium Thiosulfate:Sodium thiosulfate is generally considered non-toxic, but it can cause skin irritation in high concentrations.
- Sulfuric Acid:Sulfuric acid is a highly corrosive acid that can cause severe burns to the skin, eyes, and respiratory tract.
Safety Guidelines, Iodine clock reaction pre lab answers
To ensure a safe and successful experiment, the following safety guidelines should be followed:
- Wear appropriate personal protective equipment (PPE), including gloves, a lab coat, safety goggles, and a face shield.
- Handle iodine and sulfuric acid in a well-ventilated area.
- Never mix iodine and sulfuric acid directly, as this can result in a violent reaction.
- Use a graduated cylinder or pipette to measure chemicals accurately.
- Dispose of all chemicals and waste properly according to your institution’s guidelines.
- If any spills or accidents occur, seek immediate medical attention.
FAQs
What is the significance of the iodine clock reaction?
The iodine clock reaction serves as a valuable tool for demonstrating chemical kinetics and reaction mechanisms. Its visually striking color change makes it an engaging and accessible way to explore the concepts of reaction rates and the factors that influence them.
What are the key steps involved in the iodine clock reaction?
The iodine clock reaction proceeds through a series of steps, including the reduction of iodate ions to iodine molecules, the oxidation of thiosulfate ions to tetrathionate ions, and the subsequent reaction between iodine and thiosulfate ions to produce triiodide ions.
These steps collectively lead to the observed color change from colorless to dark blue.
How can the rate of the iodine clock reaction be controlled?
The rate of the iodine clock reaction can be controlled by varying the concentrations of the reactants, the temperature, and the presence of catalysts. Increasing the concentration of reactants or temperature will generally lead to a faster reaction rate, while the addition of catalysts can enhance the reaction rate by providing an alternative pathway with a lower activation energy.